August 5-6TH
The Cell
Summit '25
INNOVATIONS IN PROCESSING & SCALE-UP
Join us for an exclusive Thought Leadership Meeting exploring the latest innovations and challenges in
biopreservation and cell therapy manufacturing. Industry experts will discuss formulation, freezing, storage,
thawing, automation, and regulatory compliance, providing insights into optimizing workflows, ensuring
product integrity, and scaling advanced therapies. Whether you’re tackling CGT development, logistics, or
regulatory hurdles, this event offers valuable perspectives and collaboration opportunities to drive nextgeneration
cell therapy solutions.
Receive published meeting proceedings with actionable insights for
IND-enabling biopreservation success.
Join us for an exclusive Thought Leadership Meeting exploring the latest innovations and challenges in biopreservation and cell therapy manufacturing. Industry experts will discuss formulation, freezing, storage, thawing, automation, and regulatory compliance, providing insights into optimizing workflows, ensuring product integrity, and scaling advanced therapies.
Whether you’re tackling CGT development, logistics, or regulatory hurdles, this event offers valuable perspectives and collaboration opportunities to drive nextgeneration cell therapy solutions.
Receive published meeting proceedings with actionable insights for IND-enabling biopreservation success.


Meeting Details

Day 1
August 5th
8:30am – 5:00pm
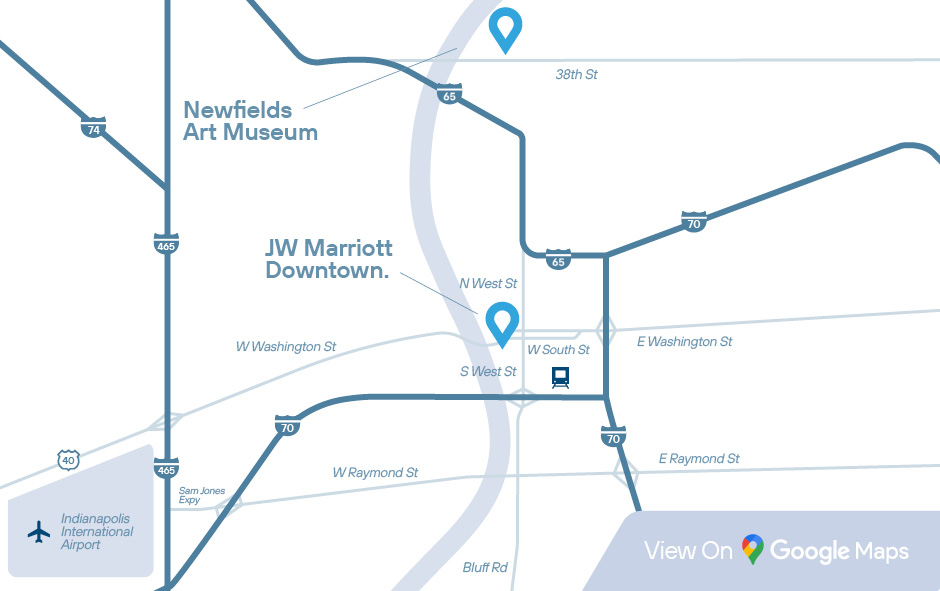
Newfields Art Museum
Day 2
August 6th
8:30am – 1:30pm
Newfields Art Museum
Accommodation
& Venue
Rooms are being held at the
JW Marriott Indianapolis.
This is a 20-minute ride from the airport.
Transportation from the hotel to/from
meetings and activities is included in your
registration. All other travel expenses
must be covered personally.

Registration Form


Speaker Panel


Aby J. Mathew, PhD
BioLife Solutions
Dr. Aby J. Mathew is a recognized thought leader in biopreservation for clinical applications, with extensive expertise in cell and tissue preservation technologies. He holds a B.S. in Microbiology and a PhD in Cell & Molecular Biology and is a co-developer of the industry-leading HypoThermosol® and CryoStor® biopreservation media. A driving force in the regenerative medicine field, Dr. Mathew has been instrumental in advancing the adoption of clinical-grade biopreservation solutions. His contributions include six issued and six pending patents, along with numerous peer-reviewed journal articles that continue to shape best practices in the industry.

Alex Sargent, PhD
Charles River Laboratories
Alex Sargent – better known as “Sarge” – is currently the Director of Process Development of Cell and Gene Therapy at Charles River Laboratories. He obtained his PhD from Case Western Reserve University in Cleveland Ohio, where he studied the challenges and promises of stem cell biology, neuroimmunology, and Cleveland sports teams. He then went on to the Lerner Research Institute at the Cleveland Clinic Foundation to continue his research in stem cell biology and neural regeneration. Since joining the biotech industry, he has worked at several large companies on drug discovery and the research and development of groundbreaking cell and gene therapies. These include Lonza Inc., where he patented new technologies for cell therapy manufacturing and CRISPR gene editing, and AstraZeneca, where he worked to bring new chimeric antigen receptor (CAR) T-cell therapies into clinical trials. He is passionate about the challenge of curing cancer, working on cell and gene therapy process and analytical development from discovery, through regulatory submission, manufacturing, and clinical trials. He wakes up each day excited to help advance cell and gene therapy to treat and cure disease, with the steadfast goal of improving human lives.

Alireza Abazari, PhD
BioLife Solutions
Dr. Alireza Abazari is currently the Senior Director of Cell Processing at BioLife Solutions. In this role, his leadership is in expanding BioLife Solutions capacity to assist customers and clients with product adoption. A deep understanding of biopreservation principles as well as hands-on experience in CAR-T and iPSC process development from his past roles at Pluristyx Inc. and Lyell Immunopharma, enables Alireza in his current role to offer a unique perspective to BioLife customers and clients, as well as to support BioLife’s Executive Team in navigating growth opportunities in the ever-evolving field.
Dr. Abazari received his PhD in Chemical Engineering from the University of Alberta where he studied methods for cryopreservation of human tissues for banking. He further studied biopreservation as a Postdoctoral Research Fellow at Harvard Medical School. During his first tenure at BioLife Solutions as Scientific Applications Director and Senior Application Scientist, Alireza drove R&D initiatives, customer consulting, and process optimization for clients. He has authored more than 30 peer-reviewed journal articles, industry white papers, and abstracts on the topic of cryopreservation and process development.
We are thrilled to have him back on BioLife Team as he continues to train and educate the sector and bring innovative solutions to cell and gene therapy process development and manufacturing.

Andrew Hamann, PhD
InVitria
Andrew Hamann is a Product Application Scientist at InVitria, where he leads internal and collaborative studies supporting the use of recombinant, animal-origin-free proteins in cell and gene therapy applications. He has experience in optimizing cell processing workflows—including cryopreservation, recovery, and expansion—with a focus on T cells and other clinically relevant cell types. Prior to joining InVitria, Andrew served as a Research Assistant Professor at the University of Nebraska–Lincoln, where his work centered on nonviral gene delivery for cell and gene therapy applications. He holds a PhD in Biomedical Engineering and is passionate about developing scalable, defined solutions that improve the safety and efficacy of advanced therapies.

David Lewandowski
phasetwo
David Lewandowski is a business development leader with over two decades of experience in the cell and gene therapy, biobanking, and life sciences industries. David is currently working as a Senior Advisor to phasetwo cryogenic storage solutions. He is also currently the Managing Director at Cells FX, where he focuses on strategic partnerships and business growth. Previously, he served as Director of Business Development for Advanced Therapies at AmplifyBio, helping to expand services for drug discovery and manufacturing. David has also held leadership roles at Azenta Life Sciences, Cryo Bio System, and Genentech, specializing in marketing strategy, commercial development, and strategic partnerships. He has contributed to industry organizations as Co-Chair of the ISCT Cold Chain Working Group and President of ISBER (2018-2019). With a deep understanding of the cryopreservation, biobanking, and regenerative medicine landscape, David continues to drive innovation and collaboration across the life sciences sector.

Donnie Beers
Entegris
Donnie Beers, Life Sciences Applications Leader for Entegris, Inc. has held many roles in process science and bioproduction over the last two decades and brings a wealth of collaboration and leadership experience gained during his past process sciences and commercial roles. Donnie joined Entegris in 2019 as Sr. Product Manager for single-use products and has since taken a lead role in in helping customers overcome unique challenges in cell and gene therapies leveraging his prior work in developing, implementing, and commercializing single-use and automation technology in biopharma. Donnie earned his BSc. in Biochemistry from University of Wisconsin – Madison.

Erik Woods, PhD
Ossium Health
Erik Woods, PhD, is a recognized leader in cell therapy and biopreservation, with extensive expertise in cryopreservation, regenerative medicine, and translational research. He has played a pivotal role in advancing cell manufacturing, biobanking, and clinical applications for over two decades. Dr. Woods has held leadership positions across academia and industry, contributing to the development of novel preservation technologies and process optimization for cell and gene therapies. His work has influenced best practices in cell storage, transport, and viability, ensuring the successful delivery of advanced therapies to patients. Passionate about innovation and collaboration, Dr. Woods continues to shape the future of biopreservation and cell-based medicine through scientific research, technology development, and industry partnerships.

Jason Acker PhD
University of Alberta/PanTHERA
Dr. Jason Acker is a distinguished leader in biopreservation, transfusion medicine, and cell therapy manufacturing, with extensive experience bridging scientific research and industry innovation. He has dedicated his career to advancing cryopreservation technologies, blood component processing, and regenerative medicine applications. With a strong background in academic research, regulatory compliance, and technology development, Dr. Acker has contributed to improving the quality, safety, and efficacy of cellular therapies worldwide. His work has led to significant advancements in biobanking, process optimization, and cold chain logistics, ensuring the integrity of biological products from lab to patient. Through his leadership, mentorship, and scientific contributions, Dr. Acker continues to shape the future of cell therapy and biomanufacturing.

Jason Jones
Cellular Origins
Jason Jones has nearly three decades of experience in biotech, with over 21 years focused on Cell and Gene Therapy (CGT) and the technologies behind processing and manufacturing. He spent 18 years at Miltenyi Biotec, leading cell therapy efforts in the UK and Ireland and later managing global strategic partnerships with companies like Autolus and GSK. He was instrumental in launching the Prodigy platform and expanding Miltenyi’s role across academia, clinical, and industry settings.
As a founding member and Chief Business Officer at Ori Biotech, Jason helped raise £135M and develop scalable CGT manufacturing technology. He briefly served as Head of Business Development at adthera bio, a UK-based CDMO, before joining Cellular Origins as Global Business Development Lead. There, he is driving innovation in full automation and robotics for CGT manufacturing, building strategic partnerships, and helping bridge the gap between CGT’s clinical potential and large-scale patient access.

Lantz Mackey, PhD
Galapagos
Lantz Mackey is an intellectually agile and versatile Development Scientist with deep expertise in process and analytical development for T-cell and hematopoietic stem cell (HSC) therapies. With a strong strategic background in Chemistry, Manufacturing, and Controls (CMC), Lantz has successfully led programs from preclinical stages through Phase I/II clinical trials. He currently serves as Director of CAR-T Process Development at Galapagos, where he leads efforts in advancing next-generation cell therapies. Previously, he spent four years at Novartis in roles of increasing responsibility, culminating as Associate Director of Cell Therapy Development. His earlier work includes pivotal contributions at bluebird bio and a postdoctoral fellowship at the National Institute of Environmental Health Sciences (NIEHS), where he utilized CRISPR-Cas9 to study human embryonic stem cell pluripotency. Lantz earned his PhD in Molecular and Cellular Pathology from the University of North Carolina at Chapel Hill and completed a post-baccalaureate certificate program in Microbiology and Immunology at Virginia Commonwealth University. Known for his decisive leadership and collaborative approach, Lantz continues to drive impactful innovations in cell therapy development.

Leela Paris, PhD
Consultant
Leela L. Paris, PhD, was with Vertex Pharmaceuticals for over five years. She received her doctorate in Medicinal Chemistry and Molecular Pharmacology from Purdue University. Previously, Dr. Paris worked as the Laboratory Director at Cook General Biotechnology and as Global Product Manager of Automation for Cook Regentec. She has extensive experience in manufacturing and development was the Vice President of Manufacturing and Process Engineering at Vertex Pharmaceuticals and the Executive Director of MSAT focusing on automation and business processes.

Mandana Haack-Sørensen, PhD
Cell2Cure
Mandana Haack-Sørensen, MSc, PhD is a leading expert in stem cell manufacturing and translational research with more than two decades of experience in cell therapy innovation. As Director of Manufacturing at Cell2Cure, she oversees the compliant production of advanced cell-based therapies for national and international clinical trials. Her expertise spans functional cell biology, regenerative medicine, quality assurance, quality control, and the development of both manual and automated cell expansion platforms. Mandana previously held leadership roles at the Cardiology Stem Cell Centre at Rigshospitalet, where she managed stem cell production, storage, and distribution. She holds a doctorate in Health Sciences from the University of Copenhagen and a master’s degree from the University of Southern Denmark. As a published scientist with over 50 peer-reviewed articles, she is also a co-inventor on a patent for adipose-derived stem cell therapy and co-founder of Cell2Cure, a company born out of the Capital Region of Denmark.

Mark Rehse, MSc
Accellix
Mark Rehse is a seasoned professional with over 40 years’ experience serving in various research and commercial roles in the biotechnology industry. Mark graduated in 1979 with a BS from University of California at Berkeley followed by an MS from San Diego State University. He started his technical training in analytical methods and immunology at Scripps Clinic and Research Foundation followed by research roles at both Genentech and CellPro, Inc. Mark accepted his first commercial role with CompuCyte as Director of European Operations in 1996 and has since worked within the biotech industry in technical sales leadership roles at Beckman Coulter and at small start-up biotech companies. Recently, after 7 years as National Sales Manager for Thomson Instrument Company in Oceanside, CA Mark retired and spent about 6 months rattling around before he realized he really missed the excitement of the pioneering work being done in biotechnology. So, as perhaps a last stint he returned to a traditional sales role at Accellix where he is currently Senior Sales Manager for the Western US.

Matthew Hewitt, PhD
Charles River Laboratories
Matthew Hewitt, BA PhD, currently serves as Vice President, CTO Manufacturing Business Division at Charles River Laboratories (CRL) playing a critical role in driving CGT strategic vision as well as leading multiple operational initiatives across CRL’s CGT CDMO, Biologics Testing, and Microbial Solutions global network. Before joining CRL, he was Head of R&D and Clinical Development for Lonza’s Personalized Medicine Business Unit leading Cocoon platform development, a closed, automated, scalable cell therapy manufacturing solution. In addition, he executed numerous collaborations across academia and industry leveraging the Cocoon. Prior to Lonza, Matt led the Tumor Immunology and Microenvironment program at Bellicum Pharmaceuticals, focusing on improving cell therapy efficacy in solid tumors. He also led the Immunology group at the University of Pennsylvania’s Gene Therapy Program, leading and contributing to numerous AAV gene therapy programs. Matt received his BA in Molecular Biology at Goucher College while playing Men’s Lacrosse, PhD in Biophysics and Physiology from the University of Alabama at Birmingham, and completed his postdoctoral fellowship at Johns Hopkins University within the Asthma and Allergy Division.

Matthew Branch, PhD
King's College of London
Matthew Branch is a seasoned researcher in ocular stem cell therapy with over 15 years of experience advancing regenerative approaches for corneal and retinal repair. Currently serving as a Postdoctoral Research Associate at King’s College London, Matthew leads a multidisciplinary team focused on developing GMP-compliant stem cell manufacturing processes for early-phase clinical trials. His expertise spans adult and pluripotent stem cell biology, cell culture, molecular biology, and flow cytometry. He received his PhD in Mesenchymal Stem Cells & Ocular Surface from the University of Nottingham and his MSc in Molecular Medicine from The University of Sheffield. Prior to his role at King’s, he held research and technical positions at UCL, where he also managed a flow cytometry core, and at the University of Nottingham. Matthew’s work continues to bridge cutting-edge research and clinical application, driving innovations in cell therapy for vision restoration.

Olga Bukatova, PhD
Azenta Life Sciences
Olga Bukatova brings over a decade of experience driving innovation in GMP manufacturing for Cell and Gene Therapies. Her expertise spans key areas such as process automation, cryopreservation and thawing, isolator technologies, and aseptic fill & finish. Passionate about making advanced therapies accessible to patients, Olga has collaborated with start-ups, public institutions, pharmaceutical companies, and CDMOs. As a member of the ISCT Cold Chain Working Group and co-host of the Bridging the Gap webinar series, she remains deeply engaged in tackling the complex challenges facing the CGT industry.

Rui Li, PhD
Consultant
Rui Li, PhD is cryobiologist and strategic generalist dedicated to bridging across organizations and empowering innovators in advanced therapies with adaptive preservation toolsets. With a PhD in Biomedical Engineering from the University of Minnesota, she has led R&D initiatives across academia, start-up, and corporate settings and has been instrumental in industry working groups shaping the future of advanced therapy manufacturing. As the founder of EastWind CryoWorks, she currently consults for startups, non-profits and corporations developing cutting-edge cellular and cold chain technologies while navigating the evolving regulatory landscape.

Sean Werner, PhD
BioLife Solutions
Sean Werner is the Chief Technology Officer at BioLife Solutions, a leading provider of bioproduction tools and services to the cell and gene therapy and broader biopharma markets. BioLife acquired Sexton Biotechnologies in 2021 where Sean was President of the company known for providing processing and handling solutions for the CGT industry. Sean received his PhD from Purdue University in Biology followed by post-doctoral positions at the Indiana University School of Medicine and Eli Lilly. Sean has previous experience filling various roles in the scientific, global regulatory, and general management functions supporting medical devices, autologous cell therapy, and single use disposable development programs. In his 23 years working in the life science industry, he has guided regenerative medicine research programs, pre-clinical and clinical testing and submission strategies leading to global commercialization of medical devices and bioprocessing tools and successful initiation of multi-national cell therapy clinical studies.

Steven Thompson, PhD
BioLife Solutions
Steven Thompson is a results-driven leader in cell therapies, regenerative medicine, and bioproduction tools, currently serving as Vice President of Sales at BioLife Solutions. With a PhD in Stem Cell Biology from the University of Liverpool, he combines scientific expertise with commercial acumen to drive sales, product development, and strategic partnerships. Previously, he co-founded Sexton Biotechnologies, leading its growth until its acquisition by BioLife Solutions. His career includes roles at Cook Regentec and Sigma-Aldrich, where he specialized in business development, sales strategy, and product commercialization. Passionate about advancing bioproduction technologies, Steven is dedicated to optimizing cell and gene therapy manufacturing to improve therapeutic outcomes.

Agenda Topics

TOPIC
Implementation of Biopreservation Best Practices in Cell and Gene Therapy Manufacturing
TOPIC
Best Practices in Thawing to Maximize Cell Recovery and Function
TOPIC
Enhancing T Cell Recovery Post-Thaw: Combinatorial Effects of HPL and Defined Supplements
TOPIC
Integrated Automation in Manufacturing to Improve CGT Scalability
TOPIC
Process Optimization Considerations:
Storage and Controls
TOPIC
Bringing the Power of Automated Flow Cytometry to the Cell Therapy Manufacturing Suite
TOPIC
Innovative Container Solutions for Efficient Downstream Processing
TOPIC
Navigating Regulatory Challenges around Formulation and Cryopreservation
TOPIC
Process Optimization: Weighing Build vs. Buy in CGT Manufacturing
TOPIC
Choosing the Right Cryogenic Packaging: Impacts on Process and Outcomes
TOPIC
Preserving the Promise:
Unique Challenges When Cryopreserving Starting Materials
TOPIC
Post-Thaw
Viability/Genomes
TOPIC
Mastering Formulation and Freezing for Optimal Cell Viability
TOPIC
Automated Storage Solutions: Enhancing Efficiency and Reliability

THE CELL SUMMIT OBJECTIVE
This meeting goes beyond simply discussing industry challenges—it is a collaborative forum focused on solutions, best practices, and actionable insights derived from real-world experience. Through expert-led discussions, we aim to drive innovation, optimize processes, and advance the field of cell therapy manufacturing.
MEETING OUTCOMES
Attendees will gain valuable insights from a published proceedings document summarizing key discussions, solutions, and best practices shared during the meeting. Beyond knowledge exchange, this event fosters new connections and first hand exposure to innovative approaches being successfully implemented across the industry.

Sponsors


Azenta life sciences is dedicated to enabling life sciences organizations around the world to bring impactful breakthroughs and therapies to market – faster.

Enabling the Technologies that Transform the World

Evening
Event Details

On Tuesday, August 5th, join us for an exciting evening at Victory Field, where the Indianapolis Indians will face off against the Omaha Storm Chasers. The game begins at 6:35 PM, and we’ve reserved the exclusive First Base Party Terrace for our group, offering a prime viewing experience.
Located just steps away from the JW Marriott, Victory Field provides convenient access for attendees. Our private terrace will feature a catered menu of classic ballpark favorites and a selection of beers, wine and non-alcoholic beverages, ensuring a delightful experience for all.
Don’t miss this opportunity to enjoy a thrilling baseball game in a vibrant atmosphere with colleagues and friends.