Overcome the challenges of cell expansion and product preservation
Independent research demonstrates the medium and material used in sample cryopreservation impacts biologic viability. Formulation must be sterile, free from adventitious agents and qualified before use to remove susceptibility from batch-to-batch. CryoStor® and HypoThermosol® FRS solutions are recognized as the gold standard freeze and storage media, embedded in over 700+ customer clinical applications, and our platform of human platelet lysates (hPL) provide scientists with a media supplement that enables reproducible cell performance.
Human Platelet Lysates
Our pathogen-reduced human platelet lysates (hPL) provide you with a media that reduces risks and improves downstream performance over Fetal Bovine Serum (FBS) and other chemically defined media. We provide a range of products and will suggest a solution for you, dependent on the regulatory and packaging needs of your processes. And with our robust supply, you’ll have a media that not only performs – but is available when you need it most.
Cell Performance
Solutions

Stemulate®-CP
Human Platelet Lysate (hPL)
Stemulate-CP is a xeno- and heparin-free pooled human platelet lysate that is used globally in clinical stem cell and immuno-oncology applications.
Details:
- Available in 100 mL and 500 mL sizes, manufactured from outdated FDA/AABB approved apheresis platelet units
- Manufactured under an ISO 9001:2015 Quality Management System
- Typically used at 2%-10% concentrations
- Large donor pool lot sizes
- FDA Master File

nLiven PR®
Human Platelet Lysate (hPL)
nLiven PR is a GMP-ready pathogen-reduced human platelet lysate with superior performance and quality standards that offers significant advantages for cell and gene therapy manufacturing by providing a reliable and effective culture supplement that improves cell growth, reduces contamination risks, enhances consistency, and can potentially lower manufacturing costs.
Details:
- Utilizes E-Beam irradiation for pathogen reduction, meeting EU Pharmacopoeia standards
- A Biodefined composition that reduces lot-to-lot variability compared to traditional PL preparations
- Contains high concentrations of growth factors and cytokines, promoting cell growth and proliferation
- Manufactured under a certified ISO 9001:2015 Quality Management System and is FDA registered and inspected since 2011
- Manufactured in accordance with cGMP standards
- Manufactured from large donor pool lot sizes
- Available in 100 mL and 500 mL sizes
- Typically used at 2%-10% concentrations
- FDA Master File, Japan PMDA Certificate of Suitability

T-Liven PR®
Human Platelet Lysate (hPL)
T-Liven PR is a GMP-ready human platelet lysate for closed-system bioprocessing with release against a T cell growth release assay for optimal immune cell quality.
Details:
- GMP-Ready: Manufactured under a certified ISO 9001:2015 Quality Management System
- Validated pathogen-reduction technology using E-Beam irradiation
- Available in bag format
- Large donor pool lot sizes
- Manufactured from outdated FDA/AABB approved apheresis platelet units
- Used for manufacturing in US, EU, and other clinical trials globally
- Typically used at concentrations ranging from 2% to 10%
- Used in 2D and 3D expansion systems
- Pathogen reduction applied per EU Pharmacopoeia
- FDA Master File, Japan PMDA Certificate of Suitability
Integrated solutions to automate and close your workflow
BioLife Solutions portfolio of cell processing solutions optimizes and protects your cells while providing increased safety and flexibility for your manufacturing suite. Our tools are designed with the evolving nature of cell and gene therapy (CGT) manufacturing in mind as we work to help developers bring new and transformative therapies to market.
Processing &
Handling Solutions
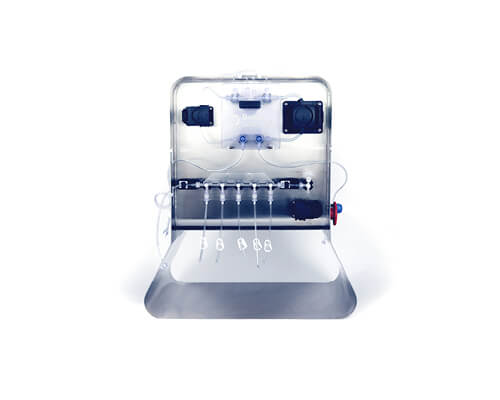
Signata CT-5®
Automated aliquoting for high-value cell products
The Signata CT-5 is a compact, benchtop aliquoting system that enables automated, aseptic filling into vials (including CellSeal® Cryogenic Vials) without the need for a biosafety cabinet. With its intuitive touchscreen interface, pre-configured fill protocols, and compatibility with closed tubing sets, the Signata CT-5 minimizes operator intervention and enhances repeatability.
Key benefits:
- Reduces cleanroom footprint
- Enhances batch reproducibility
- Lowers operator time and error
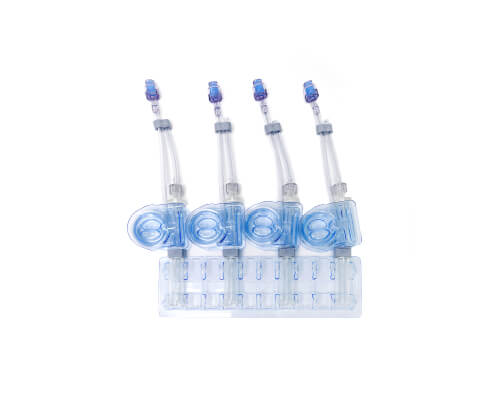
CellSeal® Connect
Seamless, closed-system transfer
CellSeal Connect advances the CellSeal platform with a weldable fill and retrieval tube. Designed for the closed-system introduction of intermediate reagents for activation, transduction, and expansion, these vials allow fill and retrieval in a closed manner via sterile welding onto downstream processing systems.
Applications:
- Mid-process sampling
- Drug product fill/finish
- Cryopreserved cell transfer
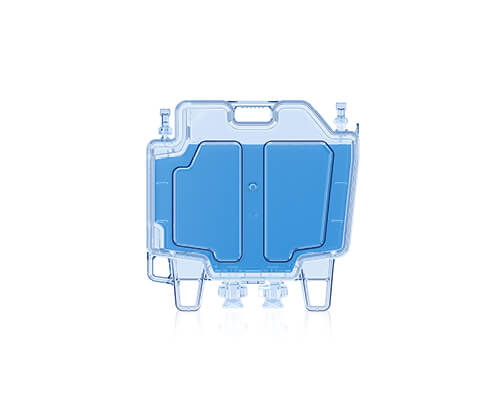
CellSeal® CryoCase
Redefining cryostorage standards
The CellSeal CryoCase is a rigid, cryopreservation container designed for CGT storage. It’s the first rigid container engineered for closed-system fill and retrieval of larger volumes of fluid (<75 mL). Its importance lies in addressing limitations of traditional cryobags, particularly the presence of particulates and the risk of leaks and fractures.
The CellSeal CryoCase has demonstrated:
- A reduction in particulates
- Improved inspectability
- Compatibility with closed-system processing
- Versatility with fill volumes
- Durability unseen before in primary container storage
The industry standard in
biopreservation media
Success in the regenerative medicine, biobanking, and drug discovery industries depends on the shelf-life and viability of cells, tissues, and organs designated for research or clinical applications. We can help you be successful with best-in-class media products for your biologic source material, intermediates, and final products.
Biopreservation
Solutions
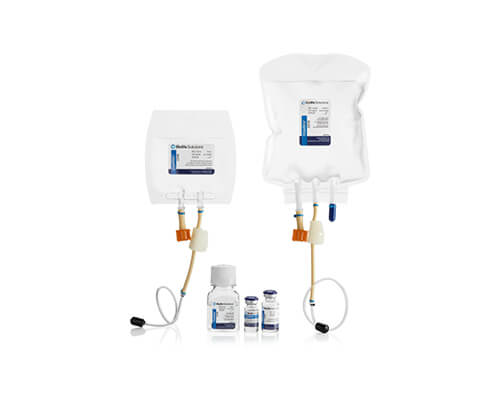
CryoStor®
Cryogenic storage
CryoStor proprietary freeze media products are intended for cryopreservation of biologics at -196°C to -70°C. Across a broad spectrum of cell types, CryoStor formulations have proven more effective in reducing post-preservation apoptosis and necrosis than commercial and home-brew isotonic and extracellular formulations.
HypoThermosol®
FRS
Non-frozen storage
HypoThermosol FRS (HTS-FRS) biopreservation media is a novel, engineered, optimized hypothermic storage and shipping media. Serum-free and protein-free, HypoThermosol is designed to provide maximum storage and shipping stability for cells and tissues at 2-8°C.
BloodStor®
Cryopreserve cells
BloodStor is a pure media product that contains various levels of DMSO concentration from 27% to 100% DMSO, in Saline or Dextran. BloodStor has been sterilized, tested for endotoxins, and manufactured under cGMPs with USP-grade components.

Cell Thawing Media
Cell and tissue thawing
BioLife’s Cell Thawing Media products originated from a Dextran shortage in the leukapheresis industry. Blood products need an added buffer for long-term storage.