In the world of manufacturing, a cell management system may track, measure, and record all work being done in a specific “production cell,” or step, within a bigger manufacturing process at a factory or plant. The main purpose of a cell management system is to improve efficiency, productivity, and flexibility within the manufacturing operation and key areas of inspection often include: automation, system integration, scheduling and planning, quality control, inventory management, maintenance and monitoring.
While a “Cell Management System” within the CGT space presents a different play on the word “cell,” many of the principals are the same. A Cell Management System refers to a set of tools, processes, and technologies used in the cell therapy manufacturing process to organize, control, and optimize the production of cells and cell therapies. A Universal Cell Management System (UCMS) should integrate with a variety of tools, fluids, or steps within any given manufacturing procedure, developed with the ability to scale up or down.
Functionally, the Signata CT-5TM meets the expectations of a UCMS due to its versatility and ability to serve various purposes within cell and gene therapy production. The following list explains how it can fulfill different functions within CGT bioproduction and the associated picture demonstrates the activity.
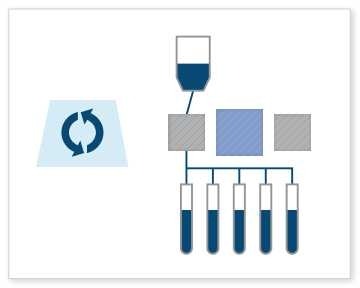 Final Fill: This is probably the most common use case. The Signata CT-5 can be used for final fill processes, which involve filling vials, cryogenic bags, or containers with the finished cell or gene therapy product. The machine will aliquot the therapy into precisely measured volumes and dispense and fill a batch of containers at one time. With fill rates reaching up to 500 mL/min, it accommodates both small and large volume fills without constraints, and its weldable connections secure one of the most crucial points in the cell therapy manufacturing process.
Final Fill: This is probably the most common use case. The Signata CT-5 can be used for final fill processes, which involve filling vials, cryogenic bags, or containers with the finished cell or gene therapy product. The machine will aliquot the therapy into precisely measured volumes and dispense and fill a batch of containers at one time. With fill rates reaching up to 500 mL/min, it accommodates both small and large volume fills without constraints, and its weldable connections secure one of the most crucial points in the cell therapy manufacturing process.
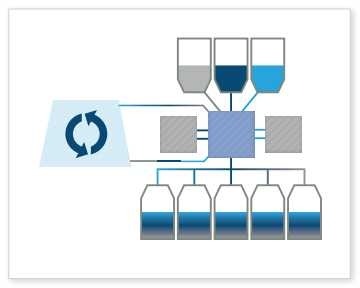 Media Formulation: The Signata CT-5 is also capable of formulating cell culture media or combining cell culture with nutrients and freeze media products to maintain optimal cell conditions for growth and viability throughout storage, shipping, manufacturing and testing. This is often done earlier in the bioproduction process and is critical to the livelihood of the cell.
Media Formulation: The Signata CT-5 is also capable of formulating cell culture media or combining cell culture with nutrients and freeze media products to maintain optimal cell conditions for growth and viability throughout storage, shipping, manufacturing and testing. This is often done earlier in the bioproduction process and is critical to the livelihood of the cell.
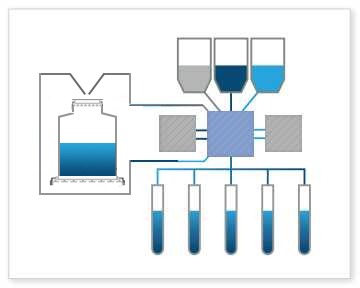
Bioreactor Fill: The Signata CT-5 can handle filling bioreactors, which are used for large-scale cell culture and production of therapeutic cell therapies. This includes adding cells, media, and other components to bioreactor systems to provide a controlled environment for enzymes or cells to transform biochemicals into products.
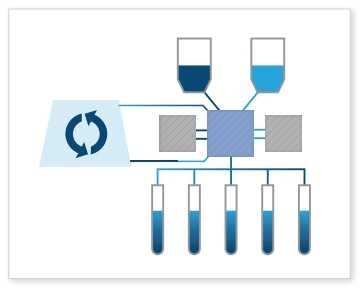 Cell Culture Wash: As a true, closed fluid transfer system, the Signata CT-5 can perform cell culture washes. This often involves washing cells to remove debris, media components, or other unwanted substances. As a fluid transfer system, the Signata CT-5 allows reversible flow direction from up to 11 input/output points and three-way control valves allowing closed movement between multiple points. Cell culture washes help maintain cell health and purity, with is paramount in the world of advanced therapies.
Cell Culture Wash: As a true, closed fluid transfer system, the Signata CT-5 can perform cell culture washes. This often involves washing cells to remove debris, media components, or other unwanted substances. As a fluid transfer system, the Signata CT-5 allows reversible flow direction from up to 11 input/output points and three-way control valves allowing closed movement between multiple points. Cell culture washes help maintain cell health and purity, with is paramount in the world of advanced therapies.To see a live demonstration of all these different fill and fluid management processes, check out this video: Signata CT-5 Fluid Management Demonstration
While many cell therapy automation systems are tailored for specific and intricate unit operations, the Signata CT-5 is engineered to revolutionize the fundamental aspects of laboratory biology by introducing flexible, closed-automation. This innovative platform aims to mitigate contamination risks, securely capture batch records, and exert precise control over fluid transfer processes, regardless of functional step or batch size.
The final detail qualifying the Signata CT-5 to truly be a Universal Cell Management System is its compatibility with a multitude of tools, tubing, primary containers, final containers, and various fluids that may be dispensed through or used with the system. The Signata CT-5 enables the utilization of off-the-shelf single-use consumables like CellSeal® Connect vials, CellSeal® Cryovials, cryobags, and other commercially available containers.
By meeting these ‘universal’ qualifiers, the Signata CT-5 demonstrates its versatility and adaptability within cell and gene therapy production workflows. Its ability to handle diverse tasks such as final fill, media formulation, bioreactor fill, and cell culture wash, highlights its role as a Universal Cell Management System, providing comprehensive support throughout the manufacturing process. With the Signata CT-5, you gain access to the first truly flexible system meticulously crafted with your team’s needs at the forefront.